
UDI & Class I Medical Devices: The obligation is now in effect. Are you ready?
As of May 26, 2025, the European Union requires UDI (Unique Device Identifier) labeling for all Class I medical devices.
An often underestimated deadline... but now unavoidable. And yet, many manufacturers are still not compliant.
This isn’t just about adding a label, it represents a major transformation of your quality, technical, and operational processes.
What is UDI?
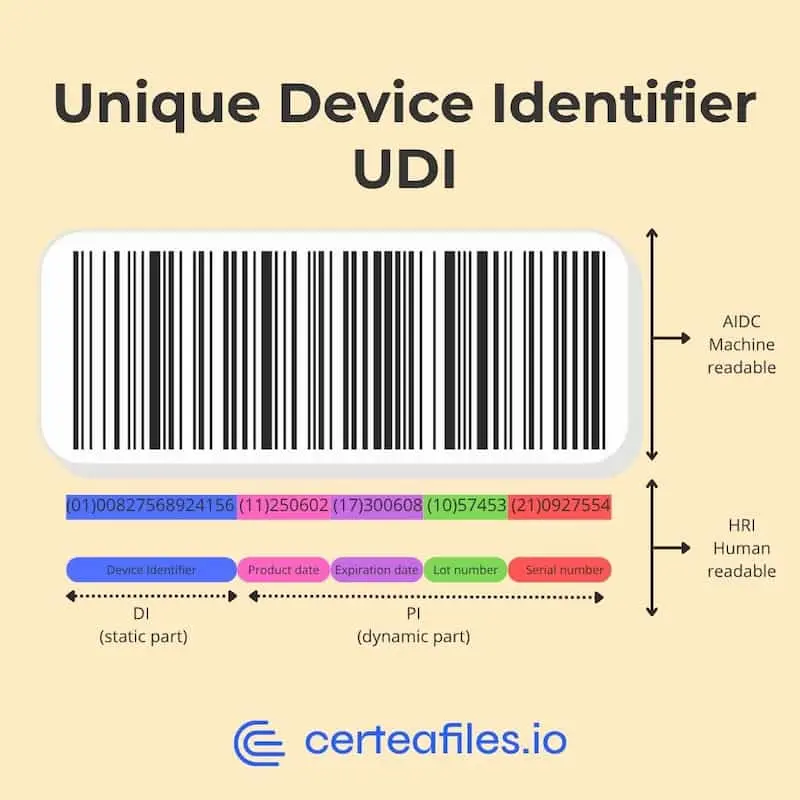
The UDI system is designed to strengthen the traceability and safety of medical devices and in vitro diagnostic devices (IVDs).
It relies on two key components:
- UDI-DI (Device Identifier): identifies a specific model of device
- UDI-PI (Production Identifier): provides data such as lot number, expiration date, or serial number
Why Does It Matter?
UDI brings tangible benefits for manufacturers, healthcare professionals, and patients:
- 📦 Improved traceability throughout the device lifecycle
- 🔁 Simplified recalls in case of issues or non-compliance
- 🛡️ Counterfeit prevention
- ❤️ Enhanced patient safety through standardized, reliable identification
Failure to comply with the UDI obligation may result in non-compliance during audits, or even block market access.
What Changes in 2025 (and Beyond)
The year 2025 marks a significant milestone in the rollout of the UDI system across multiple device categories (see article 27 of the MDR and see article 24 of IVDR).
Since May 26, 2025:
- Class I medical devices must carry UDI on their label and each higher packaging level
- Class B and C IVDs are also subject to UDI labeling
- Reusable Class IIa and IIb devices must now bear a direct marking on the device
And that’s not all:
➡️ In May 2027, obligations will extend to:
- Class A IVDs (UDI labeling)
- Reusable Class I medical devices (direct UDI marking)
ℹ️ Finally, UDI data submission to EUDAMED will become mandatory 24 months after the database becomes fully operational — a moving target, but one that must be anticipated now.
Are You Truly Ready?
Implementing UDI has direct impacts across several business functions:
- Labeling: printing UDI codes on products and packaging
- Data management: structuring and preparing data for EUDAMED integration
- QMS and documentation: updating internal procedures and ensuring regulatory alignment
- Logistics & production: adapting workflows and tools
How to Get Compliant Fast ?
If you're not ready yet, here are the key steps to initiate right away:
- Assess your current processes: QMS, labeling, technical documentation
- Review your tools: UDI code generation systems, printers, UDI databases
- Mobilize resources: internally or with expert support, especially for labeling and data management
There’s Still Time… But Not Much
UDI compliance is still achievable — if you act now, with structure and precision.
Whether you're behind schedule or just need validation of your current approach, we can help you secure your UDI strategy.
Don't wait — contact us today and let’s take action together.